ORIGINAL
Tick loads in Bos taurus cattle grazing in two contrasting production systems
Carga de garrapatas en bovinos Bos taurus que pastorean en dos sistemas productivos contrastantes
Raquel Salazar B,1,2 MV, Rolando Barahona-Rosales,1* Ph.D, María-Solange Sánchez P,3 Ph.D.
1Universidad Nacional de Colombia, Facultad de Ciencias Agrarias, Departamento de Produccián Animal, Sede Medellín,
2Centro para la Investigacián en Sistemas Sostenibles de Produccián Agropecuaria - CIPAV.
3Compañía Nacional de Chocolates, Grupo NUTRESA, Rionegro, Antioquia.
*Correspondence: rbarahonar@unal.edu.co
Received: March 2015; Accepted: December 2015.
ABSTRACT
Objective. To relate the effect of biotic and abiotic factors on Rhipicephalus (Boophilus) microplus tick loads on cows grazing either in intensive silvopastoral systems (ISS) (Lucerna) or in grass pastures associated with sugarcane plantations (La Isabela). Materials and methods. Tick counts were performed on 27 Lucerne breed animals that were in different physiological states, six of which were grazing on forage grass paddocks associated with commercial sugarcane plantations and the remaining animals grazed in an ISS based on Leucaena leucocephala and Cynodon plectostachyus. The tick counts were made every 15 days. The data of temperature, humidity, and radiation were taken from a weather station that was inside the ISS. Results. There was a weak relationship between saturation deficit and tick load (R2=0.34) and another between UV radiation and tick load (R2=0.205) for animals grazing in ISS. There were differences in tick counts when comparing animals of similar productivity from both systems evaluated: in La Isabela (sugarcane grass paddocks) average counts were 311 ticks perceptible to the touch (TPT) and in Lucerna (ISS farm) average counts were 206 TPT (p= 0.02). Additionally, there were greater tick counts in high productivity cows compared to low productivity cows. Conclusions. The abiotic and biotic factors of the ecosystem and animal productivity can affect the TPT counts. In ISS systems, tick counts can be lower than those observed in monoculture grazing systems.
Key words: Ectoparasites, microclimate, radiation, saturation deficit (Source: CAB).
RESUMEN
Objetivos. Relacionar el efecto de algunos factores bioticos y abioticos sobre las cargas de la garrapata Rhipicephalus (Boophilus) microplus en hembras bovinas que pastorean en sistemas silvopastoriles intensivos (SSPi)(Lucerna) y en monocultivos asociados a cañaduzales (La Isabela). Materiales y métodos. Se realizaron conteos en 27 animales de raza Lucerna en diferentes estados fisiolágicos, seis de los cuales se encontraban pastoreando en lotes de gramíneas forrajeras asociados con plantaciones de caña comerciales y los animales restantes pastoreaban en SSPi basados en Leucaena leucocephala y Cynodon plectostachyus. El conteo de garrapatas se efectuá cada 15 días. Los datos de temperatura, humedad y radiacián se tomaron de una estacián meteorolágica que se encontraba en el interior del SSPi. Resultados. Se encontrá una relacián débil entre el déficit de saturacián y los conteos de garrapatas (R2=0.34) y entre la radiacián UV y los conteos de garrapatas (R2=0.205) para los bovinos pastoreando en SSPi. Hubo diferencia entre los conteos en animales con similar productividad en ambos sistemas evaluados; siendo el promedio total de garrapatas perceptibles al tacto (GPT) de 311 para La Isabela y de 206 GPT para Lucerna (p=0.02). Hubo mayor número de GPT en hembras con mayor productividad en comparacián con las de baja productividad (p<0.05). Conclusiones. Los factores biáticos y abiáticos del ecosistema pueden influir en el promedio de GPT, al igual que el nivel de productividad de los animales. En SSPi, la carga de garrapatas puede ser inferior a la de sistemas de pastoreo en monocultivo.
Palabras clave: Ecosistemas, ectoparásitos, microclima, radiacián (Fuente: CAB).
INTRODUCTION
The presence of ticks on livestock farms is a global concern, given the economic and production losses that are associated with their presence in the beef and dairy industries, which amount to millions of dollars worldwide (1). These losses are due to the decrease in milk or meat production and increases in production costs associated with the control of ticks and the application of pharmaceuticals for the treatment of tick-borne diseases (2).
Ticks are poikilothermic parasites that depend on ambient temperature for their activities, with the minimum thermal threshold being 14°C (3) and optimum physiological development occurring between 27 and 32°C (4). Thus, tick growth is strongly temperature dependent, and when their need to engage in physiological adaptation activities is reduced, their reproductive ability increases. Other variables that strongly affect the survival of these populations is humidity and solar radiation by its effect on the drying of larvae and eggs (3,5,6). With increasing global temperatures predicted as part of climate change, it is expected that these arachnids are favored and can colonize new areas of life (5). The establishment of populations in new ecological zones will transfer the problems to new farmer, resulting in decreased production and increased production costs associated with tick control and treatment of blood parasites, in addition to the possible death of livestock.
The impact of abiotic factors may vary depending on the type of habitat, as a temperature of 28°C does not generate the same effect in a system with trees than in one without trees (3). This is an important consideration for intensive silvopastoral systems (ISS), production systems that promote inclusion of shrubs and trees in high densities and that have shown improvements in productivity (7,8), efficiency in use of feed resources (9) and animal welfare (10).
In an ISS, the presence of shrubs and trees in the pasture can lead to an alteration in the rate of development of Rhipicephalus (Boophilus) microplus ticks due to a variation in microclimate. Therefore, this study aimed to identify the influence of some biotic and abiotic factors on the number of tick R. (B.) microplus on cattle ISS and monoculture grassland systems located in the Valle del Cauca, Colombia.
MATERIALS AND METHODS
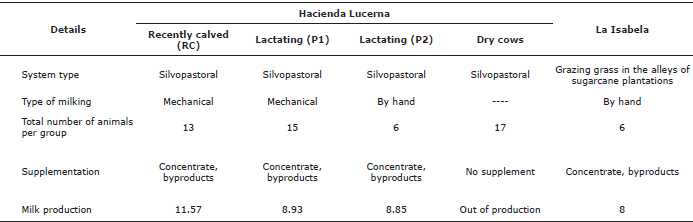
Description of the farms. The study took place in two farms located in Bugalagrande, Valle del Cauca, a site classified as tropical dry forest with 1100 mm average annual precipitation (Table 1). Both farms have milk production systems with cattle of the Lucerna breed, Colombian creole cattle generated after a series of crosses of Bos taurus cattle (Table 1). In La Isabela farm, animals grazed on the grass strips associated with sugarcane crops. In turn, the Lucerna farm is a dairy farm with 63 hectares in silvopastoral systems, comprised of the grasses star grass (Cynodon nlemfluensis) and guinea (Megathyrsus maximus) and the legumes leucaena (Leucaena leucocephala) and gliricidia (Gliricidia sepium). The Lucerna farm is also a producer of organic cane, for which allocates 160 hectares which surround the pastures for cattle grazing. In both farms, the same type of livestock is used; the Lucerna breed, which is a Colombian native cattle resulting from a series of crosses of Bos taurus breeds.
Table 1. Characteristics of the farms and animals selected for the study.
Animal selection. The selection criteria for experimental animals included choosing animals of recent entry into the different production groups, to ensure their prolonged residence time in the study. For any given group, the same number of individuals was selected. At Hacienda Lucerna, four groups were formed in total: Recently calved (RC), production group one (P1), production group two (P2) and dry cows (Dry). In this farm, and despite the initial selection, the movement of individuals within groups did not allow maintaining uniform animal group numbers throughout the study. In La Isabela, the permanence of animals within the group was favored by management, as individuals grazed in one single group regardless of productivity.
Tick counts. Tick counts were performed every fifteen days following the methodology of Wharton and Utech (11). In this technique, only the left side of the animal is used to perform the count, only counting ticks perceptible to the touch (>4mm). For greater uniformity, the animal’s body was divided into eight regions in order to reduce counting errors. These regions were: loin, ribs, abdomen, forelimb, hind limb, perianal area, armpit and udder. Tick counts were performed every fortnight in collaboration with the staff of the farm.
Calculation of forage biomass availability. Quantification of fodder availability from the ISS was performed according to the double sampling method (12). A modification of the same method was used to quantify biomass from Leucaena. In short, three one-meter rows representing different levels of growth of Leucaena (high, medium, low) were defined, fodder (leaves and fine stems) was harvested and weighed. Using this scale, at least 50 one-meter rows were visually scored.
Climatic data. Climatic data were obtained from a meteorological station installed inside the Hacienda Lucerna ISS in a shade-free site and any other component that could affect the measurements. The following variables were measured: temperature, humidity and radiation. Precipitation data were obtained from three rain gauges located in different sites of the hacienda. In the case of Hacienda La Isabela, the climatic data obtained from the Lucerna farm meteorological station was used, given the proximity of both farms.
The saturation deficit was calculated from relative humidity and temperature data. The formula used to calculate the saturation deficit is:
Da= qa*(Ts) - qa
Where qa= Real absolute humidity and Ts = dry bulb temperature
Data for saturation deficit and UV index were obtained from an average of 12 days before the counting day. The number of days was selected after a previous study of the life cycle of the tick R (B) microplus about the time required by ticks to reach the size to be perceptible to the touch (4mm).
Ethical aspects. This study had no implicit practices that were detrimental to the welfare of animals. Ticks counts were made in the milking pen, making sure of providing stress-free conditions for all animals. The person in charge of such counts was veterinarian, which also ensured adequate monitoring of the animal conditions.
Data Analysis. Tick loads of different groups were analyzed by analysis of variance following a completely randomized design. Duncan’s test was used for separation of the production groups means. Additionally, correlation analyses between animal tick load data and climatic variables were performed, including those with the UV index and saturation deficit calculated before tick counts.
RESULTS
Parasitic loads of R. (B.) microplus in different production groups.Throughout the study, there was variability in parasite counts among the groups evaluated, which was associated with the production group.
When calculating the count in Hacienda Lucerna, differences (p<0.05) between the RC, P1 and dry cows groups. The group of hand milking behaved atypically (Figure 1). On average, animals with greater nutritional requirements had higher counts.
Figure 1. Average tick loads (ticks greater than 4mm) in Lucerna cattle from the four study groups at Hacienda Lucerna, Bugalagrande, Valle del Cauca.
To compare the two farms, animal groups of similar characteristics in terms of production were chosen, and that meant averaging P1 and P2 counts for Lucerna and compare this average with the group average of La Isabela (Figure 2). This analysis showed statistical difference in the average counts for both farms (p=0.043).
Figure 2. Average tick loads (ticks greater than 4mm) in Lucerna cattle grazing in Hacienda Lucerna and La Isabelita, Bugalagrande, Valle del Cauca.
Relationship between some abiotic factors and parasite load of R. (B.) microplus. At Hacienda La Isabela, there was a positive correlation between cumulative monthly rainfall and tick counts (R2=0.53; Figure 3).
Figure 3. Monthly-accumulated rainfall and its relation to tick R (B) microplus loads on cattle Bos Taurus grazing on grass strips associated with sugarcane plantations, Finca La Isabela, Bugalagrande, Valle del Cauca.
UV radiation is negatively related to the average total parasite load in the Lucerna animals (R2=0.205). Although weak, this trend suggests an increase in parasite load in the days that had low UV index values (Figure 4). The UV index appears to be an opposite variable to the saturation deficit, since increased saturation deficit was associated with increased counts (R2=0.34; Figure 5).
Figure 4. Relationship of cumulative UV index during the 12 days prior to the counts with the average tick loads in Lucerna cattle grazing in a silvopastoral system, Hacienda Lucerna, Bugalagrande, Valle del Cauca.
Figure 5. Relationship of accumulated Dutch saturation deficit during the 12 days prior to the counts with the average tick loads in Lucerna cattle grazing in a silvopastoral system, Hacienda Lucerna, Bugalagrande, Valle del Cauca.
Relationship between forage supply and the frequency of acaricide baths. Figure 6 shows the availability of biomass as determined by the appraisals made in ISS for eight months. Biomass availability is shown separately for leucaena and star grass, as well as the number of acaricide baths that were carried out in the same period. At Hacienda Lucerna, the decision is to carry out the minimum possible of acaricide baths. The parasite load decreased 74.5% by the end of October and 38.8% after the bath made in December.
Figure 6. Forage biomass produced in silvopastoral systems and its possible relationship with acaricide baths, Hacienda Lucerna, Bugalagrande, Valle del Cauca.
DISCUSSION
The tick R. B. microplus generates great economic losses in livestock production, which are related to weight loss and reduced milk production, infections, blood parasites in infected animals and increased costs of acaricide baths. Negative effects can vary between animals due to the possible existence of acquired and natural resistance. In turn, the resistance depends on the animal breed, its physiological and health status (13). The greatest economic loss is associated to the reduction of milk production, reproductive problems and animal mortality. Additionally, there are indirect losses associated to the treatments used to control blood parasites and synthetic baths for tick control (14).
Parasitic loads of R. (B.) microplus in different production groups. The results of this study are similar to those reported by Salazar et al (2), in a study carried out in the municipality of Piedras, Tolima in which lower tick loads were observed in Bos indicus x Bos taurus animals grazing in an ISS as compared with monoculture pastures systems. Both studies evaluated production systems under similar climatic conditions, which corroborates the observation that in ISS, tick loads are reduced. By contrast, Navas (14), reported that the number of larvae found in soil in paddocks with trees had no differences with those found in a pasture without trees. It is likely that differences between studies were due to differences in the arrangements of the systems evaluated, since the density of shrubs and trees in the pasture can generate a change in the ecosystem and affect the life cycle of the organisms that inhabit it.
In the animals of this study, much greater (at least 5x) tick counts were observed than those observed in the Hacienda el Chaco by Salazar et al (2). This could be due to differences in management and use of acaricides, plus the difference in the genetic component of animals evaluated in both studies.
It is important to remember that the tick R. (B.) microplus is commonly called the cattle tick, as it presents a special predilection for animals of Bos taurus breeds, which show greater granulocytic reaction to tick infestations (15), making the Lucerna animals susceptible to this ectoparasite. Likewise, six genes have been identified that provide tick resistance to Gyr x Holstein cattle and are able to offer protection in different seasons (16). Therefore, the animal genetic component affects the impact of tick infestations in a herd, having been reported that Bos taurus cattle and their crosses are less tolerant of ticks and require more intense and costly control programs than Bos indicus cattle (17).
Relationship between some abiotic factors and parasite load of R. (B.) microplus. The growth rate of a population of poikilothermic organisms such as ticks depends on thermodynamics, that is, at greater temperatures greater are the possibilities of reproductive success (3). Now, due to global warming, the chances of ticks colonizing new biological niches, as the conditions change. Therefore, the greater the temperature, the greater the ability of these arachnids to migrate to new areas (3,18). However, interaction with other biotic and abiotic factors influence the development of non-parasitic phase of R. (B.) microplus.
The altitudinal gradient is the abiotic component most limiting to tick populations (19), since affects the temperature at which the tick grows especially during the non-parasitic phase of its cycle. However, with global warming, the restrictions associated with altitude may soon disappear (20). Another important factor is soil drainage, as flooding can be a limiting factor for the growth of tick populations (18). In the study of Adejinmi (21), it was concluded that female ticks exposed to long periods of immersion in water reduce their reproductive capacity. Most pastures where our study took place had good drainage, so this factor was not important to explain our results.
Rain is important for the survival of ticks. When rainfall is accompanied by temperatures below 14°C, the ability of ticks to oviposition is altered (22). However, the rain is important for the survival of these ectoparasites, as immediately after precipitation there is increased evapotranspiration which in turn leads to an increase in the relative humidity inside the systems (6), encouraging a suitable microclimate for tick larvae to actively search a host. This important step may not be possible during the dry season (23), because any movement of the larvae with low saturated vapor in the air could lead to desiccation. In this study, high rainfall favored high parasite loads in the group of La Isabela. Salazar et al (2) they observed a similar pattern in parasite loads in the rainy season, suggesting that the silvopastoral system can act as a buffer against some climatic variables and in turn, alter the ecology of ticks.
Increased humidity and suitable temperature inside the pasture, leads to a suitable saturation deficit, which is the amount of water needed for air moisture to condense as dew, and is calculated based on temperature and relative humidity. According to Gern et al (18), the saturation deficit is important to ticks for two reasons: 1). The larvae take water from the water vapor to avoid desiccation. 2). When water vapor is insufficient, larvae descend from the leaves to fetch water; wasting energy that could be used to survive longer.
The saturation deficit is a value frequently used in modeling the population dynamics of tick populations both to assess climate change, and to simulate the effect of rotational grazing (24, 25). From these exercises it is concluded that there is a strong relationship between mortality of larvae and eggs and saturation deficit (25). It is important to note that, after a retrospective study that took weather information from different European countries, it was concluded that there was no relationship between cumulative rain with relative humidity and saturation deficit (26). Thus, it is not appropriate to take precipitation as the only explanatory variable for tick population dynamics (26).
Another of the abiotic factors studied was radiation, which has an important effect on young organisms. Langrová et al (27) conducted a study to assess the influence of the drying effects of UV radiation in intestinal parasites in horses exposed to the sun for several days. The results were 2.5% death of larvae 3 (infective) after 17 days of exposure (27). Similar work was carried out with Tetranychus urticae, but this time aiming to assess the effect of exposure to UV rays. Brief UV exposure periods lead to larvae death and unviable eggs (28). Navas-Panadero (14) evaluated the photosynthetically active radiation and its effect on the number of larvae of ticks found in pastures with different silvopastoral arrangements and found no difference between the silvopastoral and more conventional arrangements. It is important to recognize that UV rays are not a single homogeneous variable, but have different effects according to wavelength (Table 2).
Table 2. Overview of ultraviolet (UV) rays and their biological effects (28).
In the present study there was a decrease in tick loads when radiation prior to tick counts was high. This could lead to a decrease of larvae and viable egg numbers in the pastures, thus solar radiation reduce the chances of survival of organisms (29). Future studies should include quantification of different amounts of UV rays reaching the niches occupied by ticks in both ISS and traditional systems.
There is no single variable on which to base a study of tick dynamics, making it difficult to identify one variable as the most important for the biological cycle of the tick R. (B.) microplus.
Silvopastoral systems are beneficial for both for the environment and biodiversity (7) and livestock production (8) due to increased availability and nutritional quality of forage biomass (7) and environmental conditions that reduce climate stress (10). A silvopastoral system could encourage the growth of the population of ticks R. (B.) microplus, by generating a more suitable growth habitat. However, more favorable habitat for insects and other arachnids which can act as natural tick predators are also generated, keeping the ecosystem in balance.
In conclusion, when comparing animals of the same physiological state and with a similar level of milk production, animals grazing in ISS have 56% lower tick load than that observed in a monoculture pasture system. In turn, animals with greater nutritional requirements show greater ticks loads compared with cows of other groups.
Both abiotic and biotic factors influence parasite loads, and it is not possible to identify a single variable on which to base a study of dynamics of R (B) microplus ticks, but there is an evident ecosystem effect on the population of ticks.
REFERENCES
1. Rodríguez-Vivas RI, Hodgkinson JE, Trees. Revisián: Resistencia a los acaricidas en Rhipicephalus (Boophilus) microplus: situacián actual y mecanismos de resistencia. Rev Mex Cienc Pecu 2012;3 Supl 1:9-24 [fecha de acceso 11 de marzo de 2015]; URL disponible en http://www.tecnicapecuaria.org.mx/trabajos/201210084933.pdf
2. Salazar Benjumea RS, Barahona Rosales R, Chará Orozco JD, Sánchez Pinzán MS. Productivity and tick load in bos indicus x b. taurus cattle in a tropical dry forest silvopastoral system. Tropical and Subtropical Agroecosystems 18(2015):103-112
3. Frazier MR, Huey RB, Berrigan D, Gern L, Morán Cadenas F, Burri C. Thermodynamics constrains the evolution of insect population growth rates: ″warmer is better”. Am Nat 2006; 168(4):512-20.
4. Estrada-Peña A, Bouattour A, Camicas JL, Guglielmone A, Horak I, Jongejan F, Latif A, Pegram R, Walker AR. The known distribution and ecological preferences of the tick subgenus Boophilus (Acari: Ixodidae) in Africa and Latin America. Exp App Acarol 2006; 38:219 -335.
5. Sutherst RW, Bourne AS. The effect of desiccation and low temperature on the viability of eggs and emerging larvae of the tick, Rhipicephalus (Boophilus) microplus (Canestrini) (Ixodidae). Int J Parasitol 2006; 36:193−200
6. Corson, M.S, (2004). Microclimate influence in a physiological model of cattle-fever tick (Boophilus spp.) population dynamics. Ecol Model. 2004; 180:487-514.
7. Cuartas CA, Naranjo JF, Tarazona AM, Murgueitio E, Chará JD, Ku Vera J et al. Contribution of intensive silvopastoral systems to animal performance and to adaptation and mitigation of climate change. Rev Col Cienc Pec. 2014; 27(2):76-94.
8. Murgueitio-Restrepo E, Chará-Orozco JD, Barahona-Rosales R, Cuartas-Cardona CA, Naranjo-Ramírez, JF. Intensive silvopastoral systems (ISPS), mitigation and adaptation tool to climate change. Tropical and Subtropical Agroecosystems 2014; 17(3):501−507.
9. Cuartas CA, Naranjo JF, Tarazona AM, Barahona R. Uso de la energía en bovinos pastoreando sistemas silvopastoriles intensivos con Leucaena leucocephala y su relacián con el desempeño animal. Rev CES Med Vet Zoot 2013; 8(1):70−81.
10. Tarazona AM, Ceballos MC, Cuartas CA, Naranjo JF, Murgueitio E, Barahona R. The relationship between nutritional status and bovine welfare associated with adoption of intensive silvopastoral systems in tropical conditions. Some Case Studies. Roma: FAO; 2013.
11. Wharton RH, Utech KBW. The relation between engorgement and dropping of Boophilus microplus (Canestrini) (Ixodidae) to the assessment of tick numbers on cattle. J Aust Entomol Soc 1970; 9:171-182.
12. Haydock KP, Shaw NH. The comparative yield method for estimating dry matter yield of pasture. Aust J Exp Agr 1975; (15):663-670.
13. Jonsson NN. The productivity effects of cattle tick (Boophilus microplus) infestation on cattle, with particular reference to Bos indicus cattle and their crosses. Vet Parasitol 2006; 137(1-2):1−10.
14. Navas Panadero A. Influencia de la cobertura arborea de sistemas silvopastoriles en la distribucián de garrapatas en fincas ganaderas en el bosque seco tropical. [Tesis M.Sc]. Turrialba, Costa Rica: 2003. URL Disponible en: http://www.sidalc.net/repdoc/A0116e/A0116e.pdf.
15. Constantinoiu CC, Jackson LA, Jorgensen WK, Lew-Tabor AE, Piper EK, Mayer DG, et al. Local immune response against larvae of Rhipicephalus (Boophilus) microplus in Bos taurus indicus and Bos taurus taurus cattle. Intern J Parasitol 2010; 40(7):865-875.
16. Machado MA, Azevedo, AL, Teodoro RL, Pires MA, Peixoto MG, de Freitas C, Prata MC, Furlong J, et al. Genome wide scan for quantitative trait loci affecting tick resistance in cattle (Bos taurus × Bos indicus). BMC Genomics 2010; 11:280. [Fecha de acceso 11 de marzo de 2015]; URL disponible en http://www.biomedcentral.com/content/pdf/1471-2164-11-280.pdf
17. Molento MB, Fortes FS, Buzatti A, Kloster FS, Sprenger LK, Coimbra E, et al. Partial selective treatment of Rhipicephalus microplus and breed resistance variation in beef cows in Rio Grande do Sul, Brazil. Vet Parasitol 2013; 192(1):234-239.
18. Gern L, Moran Cadenas F, Burri C. Influence of some climatic factors on Ixodes ricinus ticks studied along altitudinal gradients in two geographic regions in Switzerland. Int J Med Microbial 2008; 298(1):55-59.
19. Cortés Vencino JA, Betancourt JA, Argüelles J, Pulido L A. Distribucián de garrapatas Rhipicephalus (Boophilus) microplus en bovinos y fincas del altiplano cundiboyacense (Colombia). CORPOICA Cienc Tecnol Agropecu 2010; 11(1):73-84.
20. Estrada-Peña A, Venzal JM. High-resolution predictive mapping for Boophilus annulatus and B. microplus (Acari: ixodidae) in Mexico and Southern Texas. Vet Parasitol 2006; 142:350−358.
21. Adejinmi JO. Effect of water flooding on the oviposition capacity of engorged adult females and hatchability of eggs of dog ticks: Rhipicephalus sanguineus and Haemaphysalis leachi leachi. J Parasitol Res 2011; 11 Article ID 824162. URL disponible en http://www.hindawi.com/journals/jpr/2011/824162/cta/
22. Süss J, Klaus C, Gerstengarbe FW, Werner PC. What Makes Ticks Tick? Climate Change, Ticks, and Tick-Borne Diseases. J Travel Med 2008; 15(1):39-45.
23. Tomkins JL, Aungier J, Hazel W, Gilbert L. Towards an Evolutionary Understanding of Questing Behaviour in the Tick Ixodes ricinus. PLoS ONE 2014; 9(10):e110028. URL disponible en http://www.plosone.org/article/fetchObject.action?uri=info:doi/10.1371/journal.pone.0110028&representation=PDF
24. Hoch T, Monnet Y, Agoulon A. Influence of host migration between woodland and pasture on the population dynamics of the tick Ixodes ricinus: A modelling approach. Ecol Model 2010; 221(15):1798-1806.
25. Navas Panadero A. Importancia de los sistemas silvopastoriles en la reduccián del estrés calárico en sistemas de produccián ganadera tropical. Revista de Medicina Veterinaria 2010; 19:113-122. URL disponible en: http://revistas.lasalle.edu.co/index.php/mv/article/view/782/691
26. Alonso-Carné J, García-Martín A, Estrada-Peña A. Assessing the statistical relationships among water-derived climate variables, rainfall, and remotely sensed features of vegetation: implications for evaluating the habitat of ticks. Exp Appl Acarol 2015; 65(1):107-24.
27. Langrová I, Jankovská I, Vadlejc, J, Libra M, Lytvynets A , Makovcová K. The influence of desiccation and UV radiation on the development and survival of free-living stages of cyathostomins under field and laboratory conditions. Helminthologia 2008; 45(1):32-40.
28. Murata Y, Osakabe M. The Bunsen−Roscoe reciprocity law in ultraviolet-B-induced mortality of the two-spotted spider mite Tetranychus urticae. J Insect Physiol 2013; 59(3):241-247.
29. Paul ND, Gwynn-Jones D. Ecological roles of solar UV radiation: towards an integrated approach. Trends Ecol Evol 2003; 18(1):48-55.